23 August 2024
Updated 09 September 2024
In: Van der Linden, J. 2024. Some endophagous insects from the Upper Midwest, USA. Self-published Web reference.
Available online at https://insect-pages.github.io/reports/thrypticus/thrypticus-Upper-Midwest.html.
Overview
Larvae in the genus Thrypticus (Diptera: Dolichopodidae) are known for their phytophagous habits (see, e.g., Dyte (1993)). As part of an investigation into endophagous insects in the Upper Midwest, USA, JvdL initiated studies of Thrypticus larvae and associated feeding traces in culms of several species of host grasses (Poaceae). Larvae and their feeding sign were documented with photographs and notes, and in some cases larvae were reared to the pupa or adult stage. This report shares the results of these studies. An annotated list of known hosts for the genus is also provided.
Summary of findings
Ecology. All observed larvae were found in culms of grasses (Poaceae) in the Upper Midwest [2], USA. Host genera documented in the current study include Agrostis, Andropogon, Elymus, Muhlenbergia, Poa, and Setaria. Many inhabited plants were found close to rivers, creeks, or intermittent streams. Other inhabited plants were located away from water and belonged to plant species with a "facultative" (FAC) or "facultative upland" (FACU) wetland indicator status (USACE 2023). For example, some inhabited culms of Andropogon gerardii (FACU) were found in a remnant dry prairie on a hilltop, while inhabited culms of Poa cf. pratensis (FAC), Elymus repens (FACU), and S. pumila (FAC) were found from low- to midslope on a hillside.
While Thrypticus larvae were usually found to be unaccompanied inside grass culms, one individual was observed with an ectoparasitoid wasp larva attached to its abdomen.
Feeding sign. In grass culms having a solid interior filled with pith, such as those of Andropogon gerardii, the typical feeding sign of an early- to middle-instar larva consisted of a long linear tunnel in the pith, the tunnel round in cross-section with ragged walls. Feeding sign in hollow culms varied somewhat according to the host and the age of the larva. In Elymus, early- to middle-instar larvae formed raised linear tunnels in the thin layer of ground tissue lining the inner walls of the culm. The raised linear tunnels in the inner culm walls did not generally run the entire length of an internode; rather, each one covered only part of the length of the internode, and multiple tunnels would often be found running parallel to one another along the inner culm walls, with the ends of the tunnels overlapping. The raised roofs of the tunnels were very thin and would often develop cracks or gaps, and photos of some of these tunnels showed them to be roughly oval in cross-section. In hollow culms of certain hosts, larvae were sometimes accompanied by long filaments of plant tissue apparently related to larval feeding but whose exact origins were mysterious. In most hosts, older larvae could be found loose in the central cavity of the culm near a node, ensconced in a collection of coarse debris apparently generated by their feeding activities.
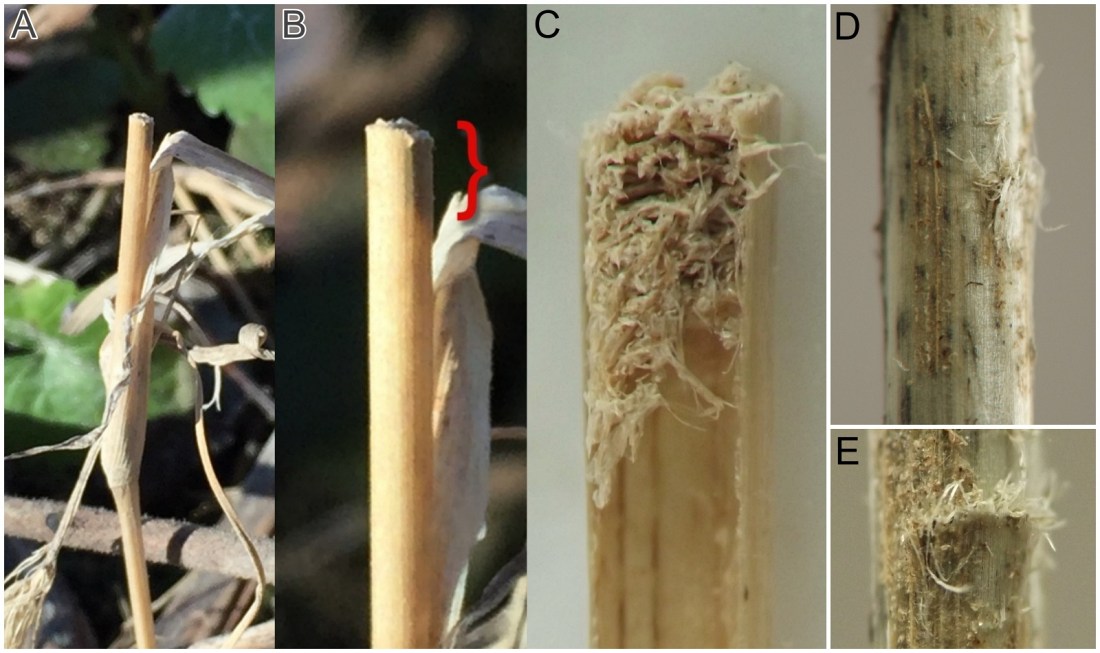
Overwintering. In some cases, a larva was found overwintering just above a node in the culm, surrounded by debris as aforementioned. In other cases the overwintering location was a chamber in the lowest part of the culm at or just above ground level. Sometimes the culm just above the upper end of such a chamber was cut neatly by the larva around its circumference, causing the culm above this point to break off, and leaving behind a lower stem stump with the larva inside in its overwintering chamber. In these cases the open top of the stump was plugged with a cap of frass. Many such stumps were prepared in autumn, prior to overwintering, but others did not become visible until late winter or early spring.
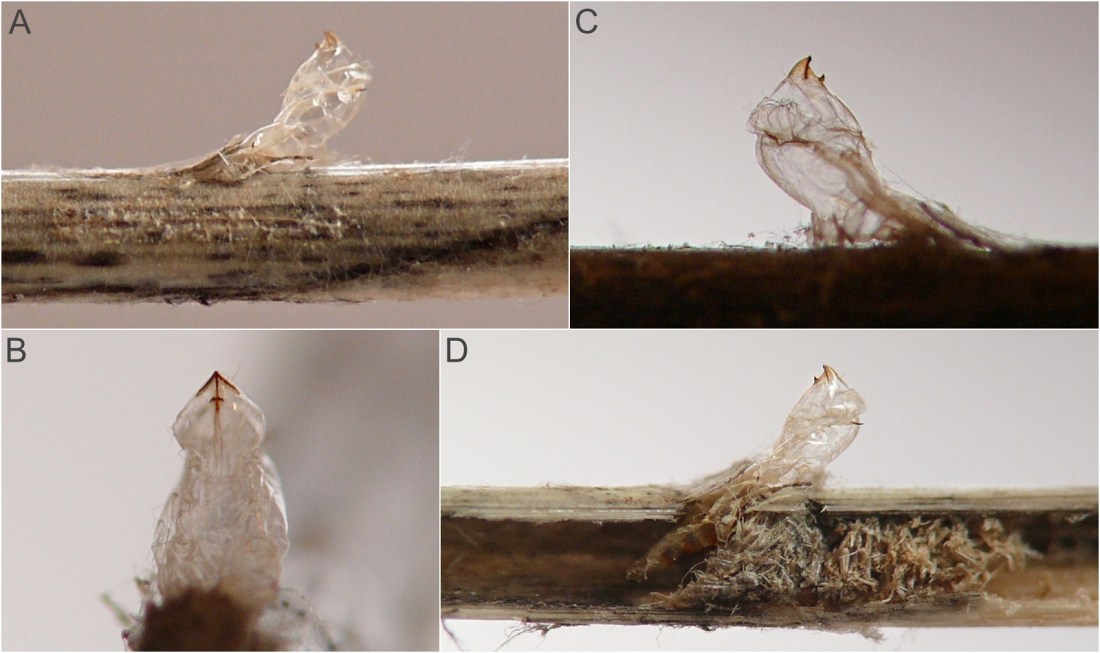
Pupation. All larvae who were observed to pupate did so in spring after overwintering in the culm. These larvae generally pupated in a chamber in the culm whose central portion was more or less cleared of debris, but whose upper end was plugged with a cap of frass. Just prior to pupation, the larva cut an inconspicuous exit opening in the wall of the culm just below the base of the frass cap, and then withdrew to the bottom of the chamber to pupate. When the adult was ready to emerge, the pupa was thrust partway through the exit opening in the wall of the culm just below the base of the frass cap.
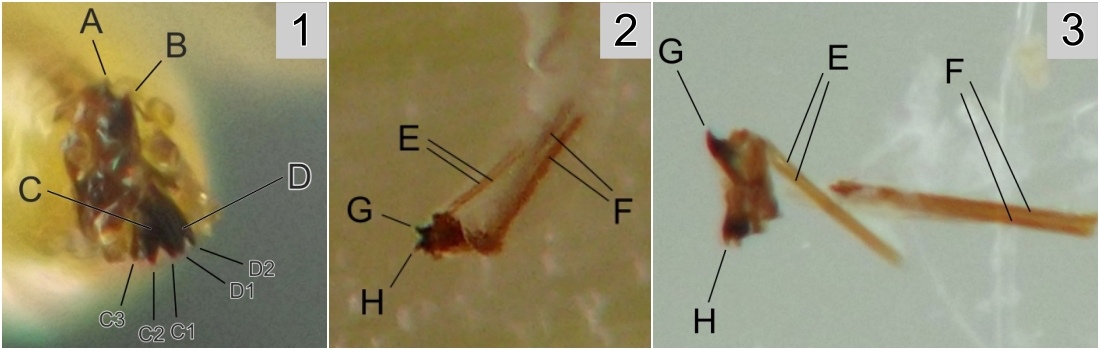
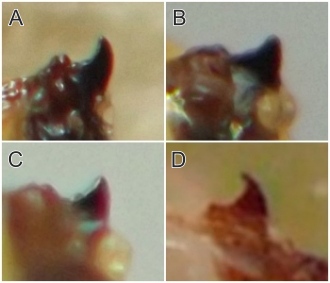
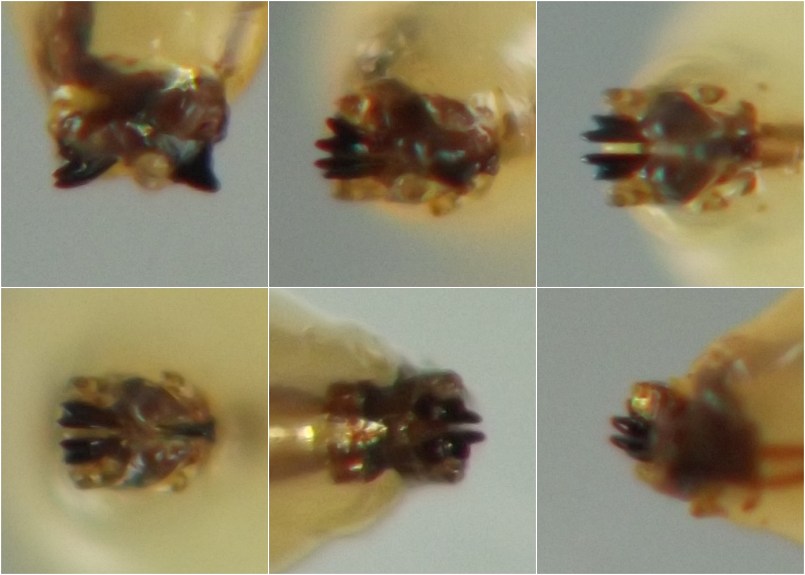
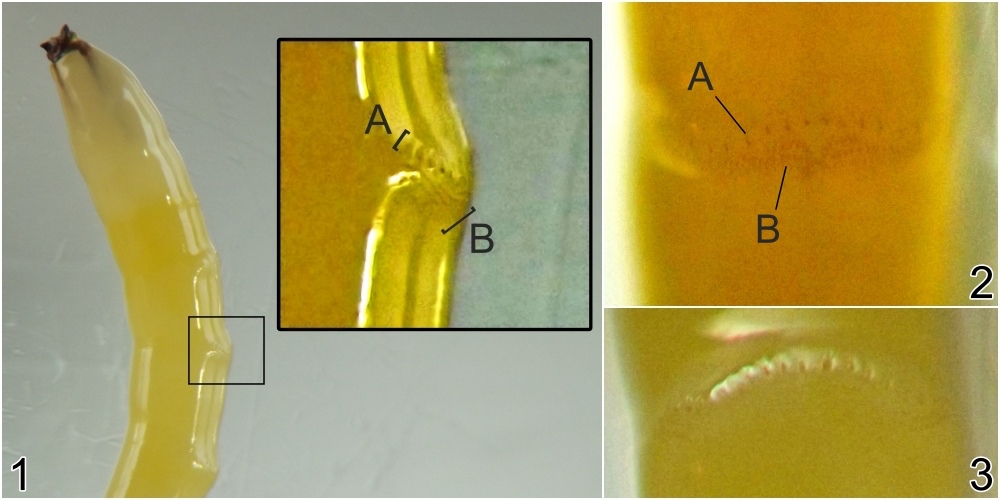
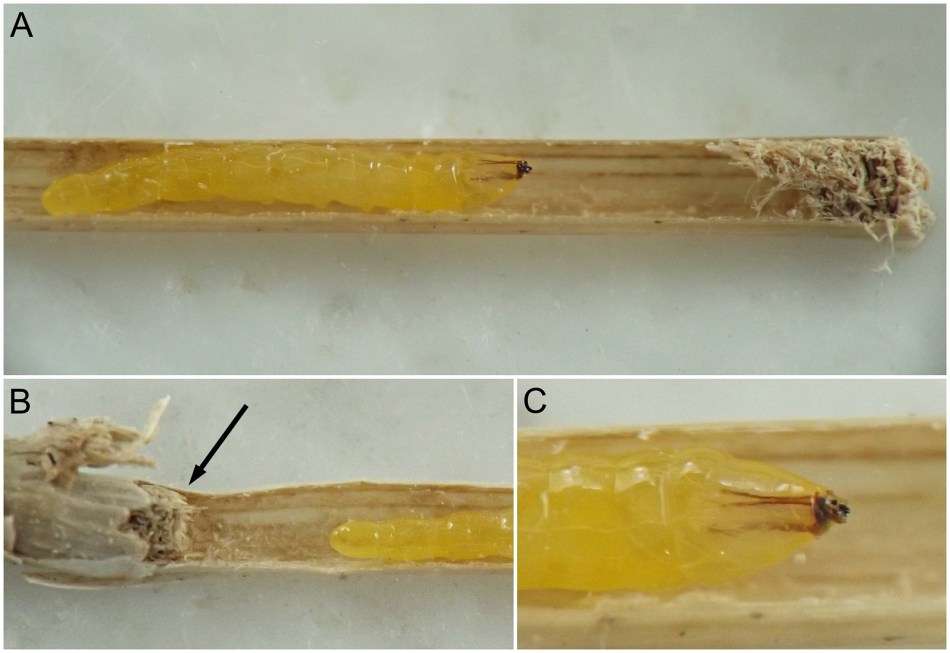
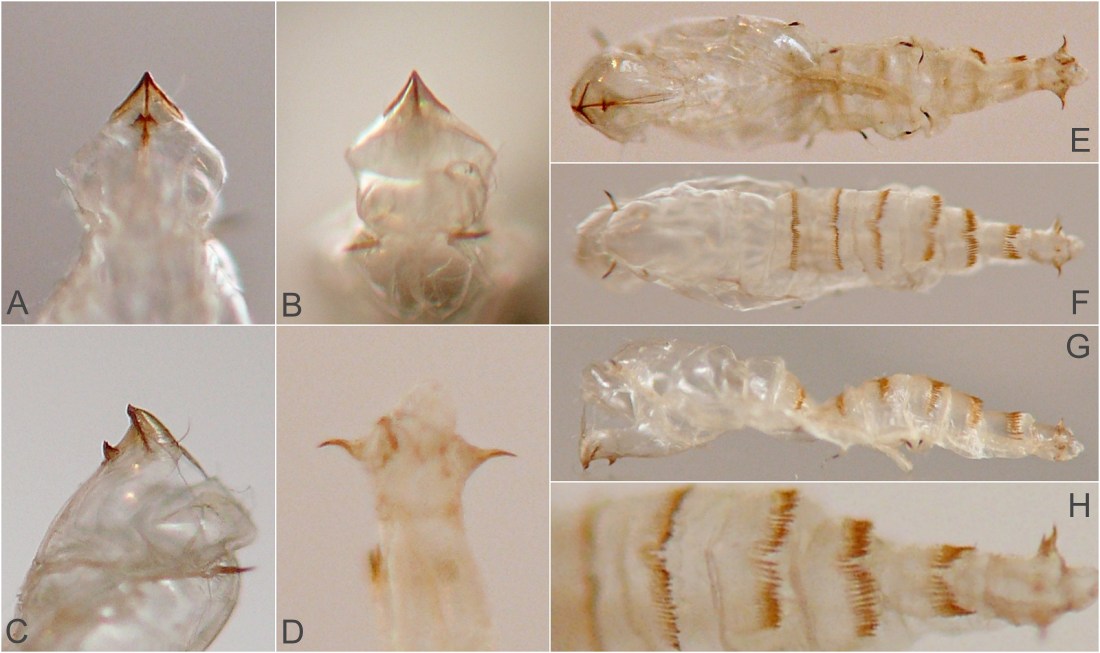
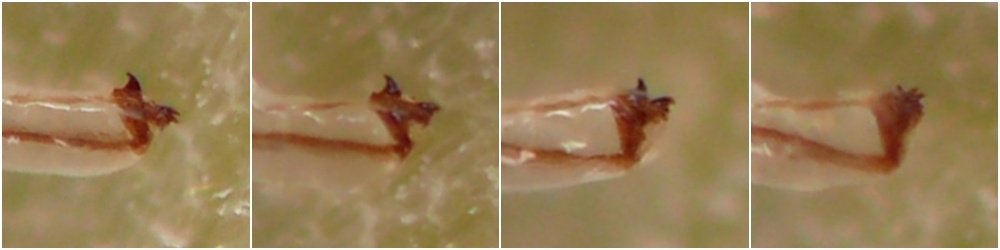
Larval morphology. Larvae could be readily recognized by the combination of their microhabitat and their unique morphology. (Terminology used herein for larval morphology is based on de Meijere (1947), Courtney et al. (2000), and Hernández (2008)).The few very young larvae observed in the current study were pale and whitish, while all older larvae encountered were a warm, sometimes bright yellow color. On the anterior end of all observed larvae, there were two prominent externally projecting elements of the head skeleton: (1) a pair of black, ventrally-pointing mandibular hooks and (2) dorsad of these, a single conspicuous, black, dorsally-pointing triangular structure, the labrum (Figs. 1 & 2). Differences in the shape of the labrum were noted in larvae of different ages and host affinities (Fig. 2; see also see Hernández (2008) for illustrations of the labrum on Thrypticus larvae from Pontederiaceae in South America). Sequential photographs of a young larva showed that both the paired mandibular hooks and the labrum are capable of movement and/or at least partial retraction into the body, with the mandibular hooks able to be drawn up until they reach the same height as the labrum. The mobility of these parts in Thrypticus larvae was also commented upon by Hernández (2008, pp. 1044-1045), who wrote, "The labrum and the mandibular hooks [of Thrypticus truncatus and T. sagittatus] can move up and down, either together or independently of each other. These three hooks are almost constantly in movement and they serve to tear the plant tissues up." In addition to these externally projecting elements of the head skeleton, internal parts of the head skeleton were visible in larvae as well, such as the paired metacephalic rods and tentorial arms (Fig. 1).
Certain morphological differences were noted between the Thrypticus larvae documented in grasses in the current study and those documented from Pontederiaceae in South America by Hernández (2008). For example, the Thrypticus larvae from grasses did not possess the conical projection on the last abdominal segment shown by larvae of T. truncatus and T. sagittatus from Pontederiaceae (Hernández 2008, figs. 1 and 5). Mature Thrypticus larvae from Pontederiaceae were described by Hernández as possessing "crystalline grayish white body contents" (ibid., p. 1044), while mature larvae from grasses in the current study were all yellow in color. Other differences noted include the quantity and arrangement of spinules on the creeping welts (compare figures 3 and 4 in Hernández (2008) with image no. 0053-T10 below under Andropogon) and the number of teeth on the mandibular hooks (the apical end of the hook divided into two leading teeth in larvae from Poaceae, but evidently undivided in larvae from Pontederiaceae). The anal pads of larvae from Poaceae appear to be similar to those of larvae from Pontederiaceae (compare fig. 6 in Hernández (2008) with panels D-E in image no. 0053-T02 under Andropogon, below).
Thrypticus ex Andropogon gerardii
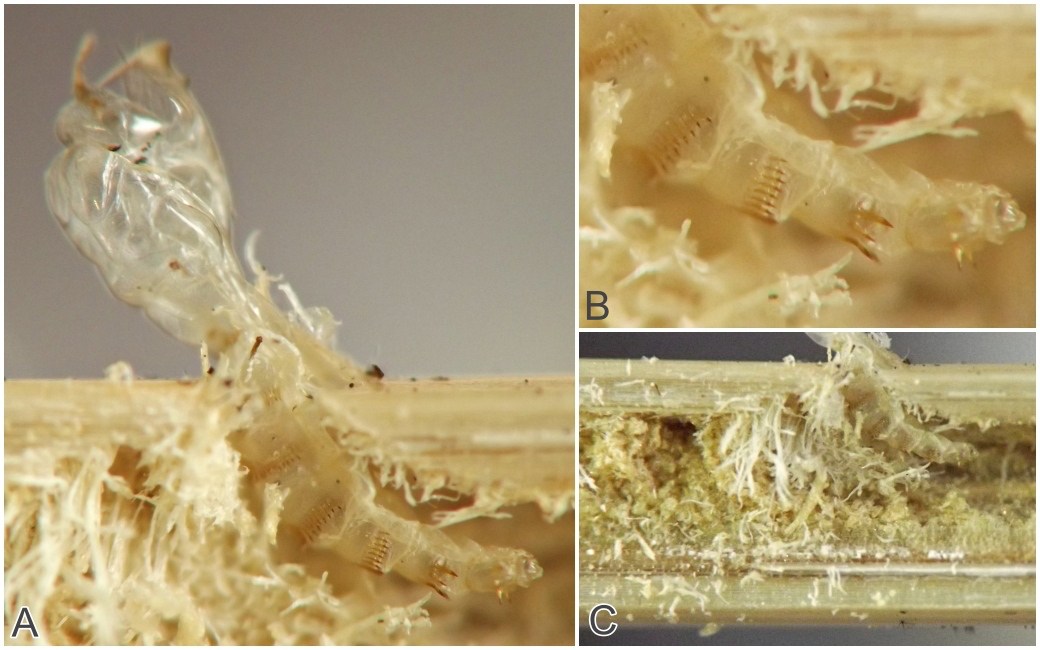
This borer tunnels in the culm of the host, at least sometimes starting fairly high in the culm (approximately 1m above the ground in one example examined in the current study). A photograph of a larva's tunnel in a dissected stem at a point several decimeters above ground level shows that the tunnel was approximately 1/8 of the culm diameter, with rough-textured walls and no significant solid frass accumulation. The late-stage larva is a warm yellow color, elongate (roughly 12 times as long as wide) and cylindrical, the body tapering little except at the very front, the posterior end rounded. The larva finishes up its feeding at the base of the plant where it passes the winter and constructs a pupation chamber in the senescent culm. In one case, the chamber was several centimeters long and it occupied most of the diameter of the culm. Upon the emergence of the adult fly, the pupa was thrust partway out of the culm. Examination of the pupal exuviae revealed transverse rows of posteriorly-directed spines on the abdominal segments, which had perhaps helped the pupa gain purchase on the inner walls of the chamber.







Thrypticus ex Setaria pumila
In early September, certain seedheads of the host were observed to have dried down prematurely. Closer inspection revealed the upper culm below each of these seedheads had been girdled by a larva tunneling shallowly in a spiral pattern. In one culm, a very young, ~1.2mm-long Thrypticus larva was found in its tunnel in the girdled area. A different culm showed two girdled areas on adjacent internodes, with each girdled area about 10mm above the nearby node. The spiral tunnel in the lower girdled area had a wider diameter than the spiral tunnel in the upper girdled area, suggesting the larva had girdled the upper internode first, then moved downward through the culm and girdled the lower internode. The larva was found just below the node associated with the lower girdled area, with a rough-walled tunnel leading from the girdled area through the solid node to the larva's location.
Additionally, overwintering larvae were located in culms in late fall and early spring. Some individuals created stem stumps, but in at least one other case, the affected culm did not break off or lodge and so there was no stump or other obvious outward sign of the larva's presence. Adults emerged in spring. The pupal exuviae of one successfully reared adult were observed and photographed in detail, revealing features such as a prominent beak or horn on the anterior end with a smaller two-toothed projection just below it (see images 0640-T06 and 0640-T07, below).





Thrypticus ex Muhlenbergia
Inhabited stem stumps were located in late autumn, in some cases with the severed upper portion of the culm still partially attached to the top of the stump, indicating the culm had been cut recently. Thrypticus muhlenbergiae was described from culms of M. sylvatica in New York by Johannsen and Crosby (1913).


Thrypticus ex Elymus
Larvae were found in culms of E. repens and E. virginicus. Inhabited culms were easily recognized by the elongate, raised tunnels on the inner walls of the culm interior. In at least one case in E. virginicus, a mature larva was found overwintering in a stem stump that had a squarely-cut top well sealed with a plug of medium-grained frass ~3mm thick. Photographs of a young larva from E. repens show that its labrum was strongly curved into a hook or claw shape. This larva was pale whitish in color, while older larvae from E. virginicus were bright yellow.
A small number of Elymus canadensis culms were searched at a site where Thrypticus larvae were present in culms of Andropogon, but no Thrypticus larvae were located inside the E. canadensis culms. In a survey of insects associated with Elymus cinereus in Idaho, Youtie et al. (1987) discovered Diptera larvae in the culms of this grass, but they stated the larvae were "probably chloropids" (p. 645), and the larvae were not reared to adulthood.



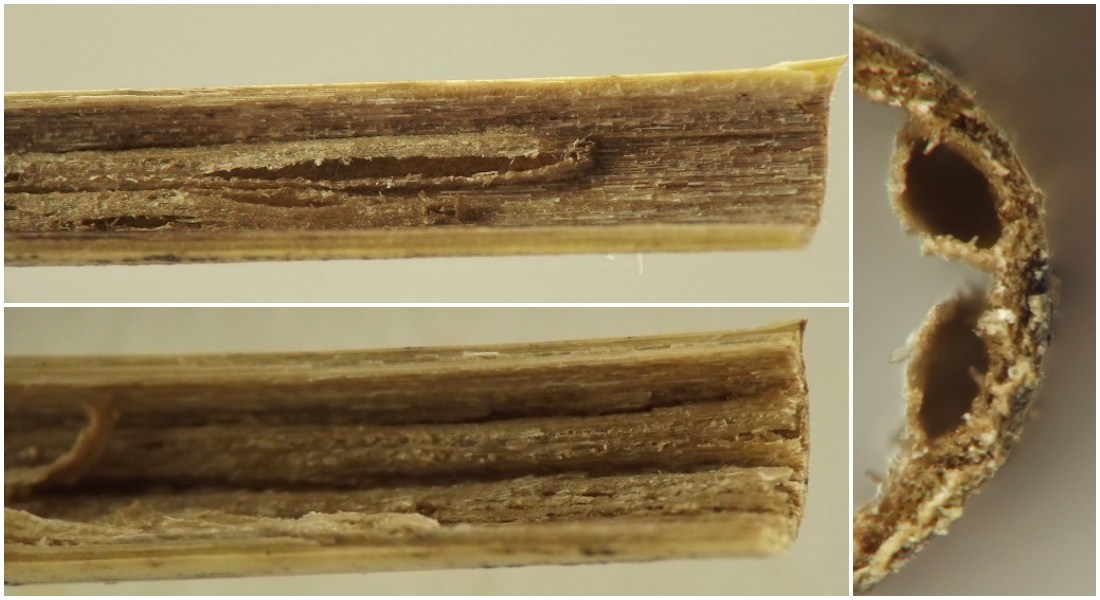


Thrypticus ex Poa
Larvae were found in the middle to lower portions of culms in late July. One individual had an ectoparasitoid wasp larva attached to its abdomen.




Thrypticus ex Agrostis
A deceased larva was found in a senesced culm of the host in winter. Its labrum was somewhat curved or hooked in shape.
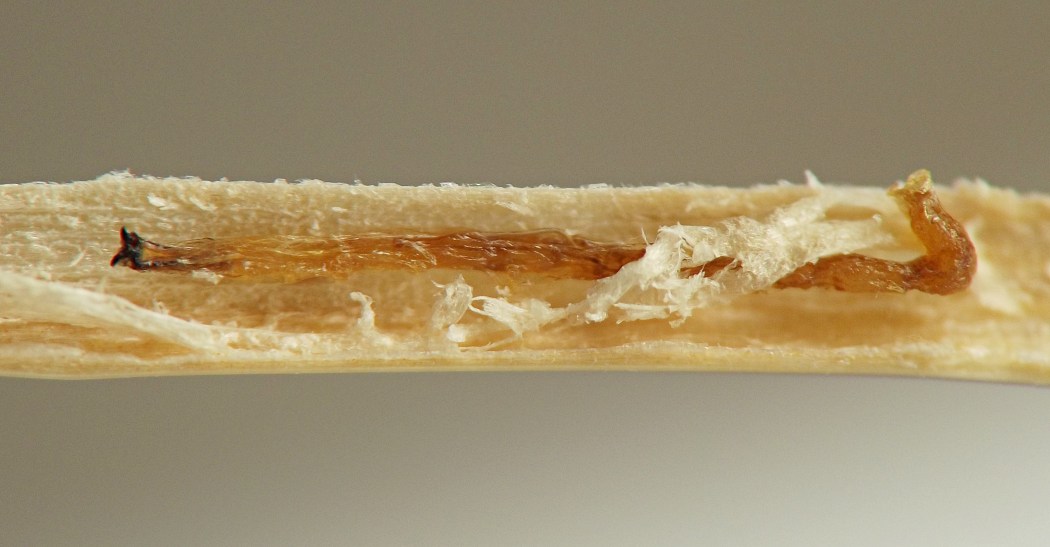

Discussion
A review of the literature suggests that some of the hosts documented above represent new host records (see "Annotated list of known host plants," below).
The finding that some inhabited plants were located away from water on slopes or even a hilltop, and belonged to plant species with a "facultative" or "facultative upland" wetland indicator status, suggests that suitable plants need not be closely associated with water or wetlands to serve as larval substrates, in contrast to what has been previously reported in the literature (e.g. Bickel and Hernández (2004) or Drake et al. (2023), but see Palmer (2016) for a report of an adult reared from an upland grass).
The parasitoid fauna of Thrypticus spp. from grass culms appears to be poorly known. The ectoparasitoid wasp larva documented in the current study unfortunately could not be reared to adulthood, but with further study, more insights may be gained.
Annotated list of known host plants
The following is a working list of host plants recorded globally for Thrypticus spp., assembled from a combination of the results of the current study and a literature review by JvdL. The list is organized by host genus. In each entry, recorded host species (if known) are listed along with the Thrypticus species involved (if identified). The geographic location of the record is also given along with the reported feeding mode of the larva (culm borer, leaf miner, etc.). Assembly of the list was facilitated by the fact that prior authors had created similar lists (see especially Bickel and Hernández 2004, Eiseman 2022 & 2024, Ellis 2001-2024a&b, Dyte 1993, and Pitkin et al. 2024a&b). Any errors or omissions are the responsibility of JvdL.
World hosts of Thrypticus1
by J. van der Linden
23 August 2024
Updated 09 September 2024
Cyperaceae
-
Eleocharis.
In Michigan, USA, an undetermined Thrypticus (referred to herein as T. sp. Michigan after Dyte (1993)) has been recorded as a miner-borer in stems of Eleocharis palustris and of the two Schoenoplectus species listed below (Frohne 1939; Eiseman 2022). In a personal communication to Drake (1999), I. Perry suggested E. palustris may serve as a host for T. cuneatus in Britain. See also: Drake et al. (2023).
-
Schoenoplectus.
In the United States, Schoenoplectus acutus has been observed to host both a stem miner-borer (Thrypticus sp. Michigan) and a leafminer (T. fraterculus), with the former record from the state of Michigan (Frohne 1939) and the latter from the state of Maryland (Greene 1954). The stem miner-borer, T. sp. Michigan, is also known to use S. americanus as a host (Frohne 1939). Eiseman (2024) reports the rearing of an undetermined T. sp. from stems of S. pungens in North Carolina, USA. Finally, Drake et al. (2023) refer to a suggestion by Negrobov and Stackelberg (1971) that T. cuneatus uses S. tabernaemontani and S. lacustris as hosts in the Palaearctic. [3] See also: Eiseman (2022).
Juncaceae
-
Juncus.
In several references, evidently first in Hering (1957), Thrypticus bellus is reported as miner on Juncus in the Palaearctic. [3] [4] De Meijere (1947) also reports a larva of an undetermined T. sp. collected from a Juncus stem in France by Dr. Herbert Buhr.
Poaceae
-
Agrostis.
An undetermined Agrostis sp. is recorded as a host for a Thrypticus sp. culm borer in the Upper Midwest, USA, in the current study (see details earlier in this article).
-
Andropogon.
A Thrypticus sp. borer was observed in culms of Andropogon gerardii in the Upper Midwest, USA, in the course of the current study (see details earlier in this article).
-
Elymus.
In the current study, both E. repens and E. virginicus are recorded as hosts for a Thrypticus sp. culm borer in the Upper Midwest, USA (see details earlier in this article).
-
Muhlenbergia.
Johannsen and Crosby (1913) described T. muhlenbergiae from specimens reared from culms of Muhlenbergia sylvatica in New York, USA. Additionally, an undetermined T. sp. was recorded from culms of M. sp. in the Upper Midwest, USA, as part of the current study. See also: Dyte (1993), Eiseman (2022).
-
Phragmites.
Lübben (1908) provided an account of the biology of Thrypticus smaragdinus, which tunnels in the interiors of shoots, rhizomes, and culms of Phragmites australis. Both T. smaragdinus and T. bellus are reported as borers in P. australis by Waitzbauer et al. (1973). Futhermore, Blossey et al. (2011) located Thrypticus larvae in culm interiors of this host in Rhode Island, and they also report larvae from the native subspecies of P. australis elsewhere in North America. They identified the Thrypticus species they encountered as T. smaragdinus, evidently through examination of reared adults. See also: Dyte (1993), Ellis (2001-2024a-b), Pitkin et al. (2024a-b).
-
Poa.
A Thrypticus sp. borer was found in culms of Poa cf. pratensis in the Upper Midwest, USA, as recorded in the current study (see details earlier in this article).
-
Setaria.
In the current study, Setaria pumila is documented as a host for a Thrypticus sp. culm borer in the Upper Midwest, USA (see details earlier in this article).
-
Spartina.
In the United States, Thrypticus larvae have been found tunneling in the interiors of both lower culm internodes and springtime shoots of Spartina alterniflora, with the shoot-boring larvae identified as T. violaceus (Newton 1984, Strong et al. 1984, Eiseman 2022). The presence of larvae in both mature stems and new shoots recalls the life history of T. smaragdinus in Phragmites (see entry above). See also: Dyte (1993).
-
Tridens.
Palmer (2016) reared an adult male identified [5] as Thrypticus violaceus from Tridens flavus. The senescent plant material from which the fly emerged included aboveground parts such as culms and inflorescences. See also: Eiseman (2022).
-
Poaceae sp., aquatic grass.
Two undetermined Thrypticus species are reported as miners in aquatic grass from the U.S. states of Louisiana and California (Greene 1954, Eiseman 2022). See also: Dyte (1993).
-
Poaceae sp., cereal grasses.
Papers by Grichanov and colleagues (e.g., Grichanov and Danielsson 2001, Khaghaninia et al. 2014) describe the habitat of Thrypticus larvae as the culm interiors of cereal grasses, but no further information is provided. In preparing this report, JvdL was unable to find any published rearing records of Thrypticus spp. from culms of cultivated grains. [6]
Pontederiaceae
-
All Thrypticus spp. reared by Bickel and Hernández (2004) were obtained from petioles or stems of their host plants.
-
Eichhornia.
An early report of Thrypticus from this host was by Cruttwell (1973), who observed flies in this genus inhabiting Eichhornia crassipes (“and possibly other Pontederiaceae”) in Trinidad. Bennett and Zwolfer (1968) reported Thrypticus from water hyacinth in Brazil, Guyana, Surinam and Trinidad [3] [7]. Furthermore, Thrypticus truncatus, T. sagittatus, T. yanayacu, T. chanophallus, and T. circularis were described by Bickel and Hernández (2004) from adults reared from E. crassipes in Argentina, Brazil, Peru, and Trinidad. The same authors (ibid.) also described T. romus and T. azuricola, reared from E. azurea in Argentina and Brazil.
-
Pontederia.
Bickel and Hernández (2004) described Thrypticus formosensis and T. taragui from specimens reared from Pontederia cordata and P. subovata in Argentina and Brazil. The species identity of a set of specimens reared by the same authors (ibid.) from P. rotundifolia in Peru is unclear.
Notes
- https://insect-pages.github.io/reports/thrypticus/thrypticus-Upper-Midwest.html
- The definition for the Upper Midwest used here is the one provided by National Centers for Environmental Information (2024), including the states of Minnesota, Michigan, Iowa, and Wisconsin.
- Bennett and Zwolfer (1968), Chandler (1978), Hering (1957), and Negrobov and Stackelberg (1971) could not be reviewed in time for the preparation of this article; they are original sources of information cited in works by other authors that were reviewed for this article. They are listed for informational purposes in the References section below, marked with an asterisk.
- Dyte (1993), who provided a table of known Thrypticus hosts, lists Hering (1957) as the original source of the Juncus record for T. bellus, and Ellis (2001-2024a) and Pitkin et al. (2024a) also cite Hering as a source for this host plant record. Ellis (2001-2024a) briefly describes the mine, but from the description it is not clear where the mine occurs on the plant. Additionally, Pitkin et al. (2024a) refer to a tentative host record of T. bellus from Juncus by Chandler (1978). See also: Cordo et al. (2000), Eiseman (2022).
- See comment by C. Eiseman, ibid., 13 July 2016
- Note, however, that one of the documented hosts for Thrypticus is closely related to cereals: Elymus is in the same tribe (Triticeae) as wheat, barley, and rye (Soreng et al. 2017).
- As relayed in Cordo et al. (2000) and Dyte (1993). The flies found by Bennett and Zwolfer in Brazil, Guyana, Surinam and Trinidad were first identified as T. insulanus (=T. minutus) (Dyte 1993, Cordo et al. 2000), but in their description of the truncatus group species listed above, Bickel and Hernández (2004) report that the specimens from Trinidad belong to T. sagittatus.
Acknowledgments
MJ Hatfield and F. Lebhardt assisted with field studies on larvae from E. virginicus, and F. Lebhardt furnished photos of a pupa and adult she and her colleagues reared. M. Hauser provided early identification assistance. A draft of this report was reviewed by C. Eiseman.
References
Bennett, F.D. and H. Zwolfer. 1968. Exploration for natural enemies of the water hyacinth in northern South America and Trinidad. Hyacinth Control Journal 7: 44-52.*
Bickel, D.J. and M.C. Hernández. 2004. Neotropical Thrypticus (Diptera: Dolichopodidae) reared from water hyacinth, Eichhornia crassipes, and other Pontederiaceae. Annals of the Entomological Society of America 97(3): 437–449.
Blossey, B., Casagrande, R. A., Tewksbury, L., Hinz, H., Hafliger, P., Martin, L., and J. Cohen. 2011. Development of biological control of introduced Phragmites australis. No. C-06-26. Cornell University.
Chandler, P.J. 1978. In Stubbs, A. and P. Chandler (eds.). A Dipterist's Handbook. The Amateur Entomologist 15.*
Cordo, H.A., Sosa, A.J., and M.C. Hernández. 2000. The petiole mining fly Thrypticus sp. (Diptera: Dolichopodidae), a new agent for the biological control of water hyacinth (Eichhornia crassipes). Pages 315-323 in Spencer, N.R. (ed.). Proceedings of the X International Symposium on Biological Control of Weeds, 4-14 July 1999, Montana State University, Bozeman, Montana, USA.
Courtney, G.W., Sinclair, B.J., and R. Meier. 2000. Morphology and terminology of Diptera larvae. Pages 85-161 in Papp, L., and B. Darvas (eds.). Contributions to a Manual of Palaearctic Diptera, vol. 1. Science Herald: Budapest.
Cruttwell, R.E. 1973. Preliminary investigations on some insects causing minor damage to water hyacinth, Eichhornia crassipes. Report, West Indian Station, Commonwealth Institute of Biological Control. 5 pp.
de Meijere, J.C.H. 1947. Over eenige Dipterenlarven, waaronder een galmug, die mijngangen maakt, en twee Dipteren, die gallen op paddenstoelen veroorzaken. Tijdschrift voor Entomologie 88: 49-62.
Drake, C.M. 1999. Two rare flies in Cambridgeshire, Ochthera manicata (Fabricius) and Thrypticus cuneatus (Becker) (Diptera, Ephydridae and Dolichopodidae). Dipterists Digest (Second Series) 6: 40-42.
Drake, C.M., Godfrey, A., and D.J. Gibbs. 2023. Thrypticus and Corindia in Britain, with the description of two new species and the addition of two species to the British list (Diptera, Dolichopodidae, Medeterinae). Dipterists Digest (Second Series) 30: 172-199.
Dyte, C.E. 1993. The occurrence of Thrypticus smaragdinus Gerst. (Diptera: Dolichopodidae) in Britain, with remarks on plant hosts in the genus. The Entomologist 112(2): 81–84.
Eiseman, C.S. 2022. Leafminers of North America, 2nd edition. Self-published e-book. Available from the author at https://charleyeiseman.com/leafminers/.
Eiseman, C.S. 2024. Leafminers of North America, 3rd edition. Self-published e-book. Available from the author at https://charleyeiseman.com/leafminers/.
Ellis, W.N. 2001-2024a. Thrypticus bellus. In Plant parasites of Europe: leafminers, galls and fungi. Retrieved August 7, 2024 from https://bladmineerders.nl/parasites/animalia/arthropoda/insecta/diptera/brachycera/dolichopodidae/thrypticus/thrypticus-bellus/.
Ellis, W.N. 2001-2024b. Thrypticus smaragdinus. In Plant parasites of Europe: leafminers, galls and fungi. Retrieved August 7, 2024 from https://bladmineerders.nl/parasites/animalia/arthropoda/insecta/diptera/brachycera/dolichopodidae/thrypticus/thrypticus-smaragdinus/.
Frohne, W. C. 1939. Biology of certain subaquatic flies reared from emergent waterplants. Papers of the Michigan Academy of Science, Arts and Letters 24: 139-147.
Greene, C. T. 1954. Larva and pupa of Thrypticus fraterculus (Wheeler) with new original notes on the habits of the family Dolichopodidae (Diptera). Entomological News 65(4): 89-92.
Grichanov, I.Y. and R. Danielsson. 2001. Dolichopodidae (Diptera) new to the Swedish fauna. Entomologisk Tidskrift 122(3): 131-134.
Hering, E.M. 1957. Bestimmungstabellen der Blattminen von Europa: Einschließlich des Mittelmeerbeckens und der Kanarischen Inseln. Springer-Verlag, 2013. 1387 pp.*
Hernández, M.C. 2008. Biology of Thrypticus truncatus and Thrypticus sagittatus (Diptera: Dolichopodidae), petiole miners of water hyacinth, in Argentina, with morphological descriptions of larvae and pupae. Annals of the Entomological Society of America 101(6): 1041-1049.
Johannsen, O.A. and C.R. Crosby. 1913. The life history of Thrypticus muhlenbergiae sp. nov. (Diptera). Psyche: A Journal of Entomology 20: 164-166.
Khaghaninia, S., Gharajedaghi, Y., and I. Grichanov. 2014. A contribution to the knowledge of the family Dolichopodidae (Diptera) in East Azerbaijan province of Iran. Check List 10(3): 588-593.
Lübben, H. 1908. Thrypticus smaragdinus Gerst. und seine Lebensgeschichte: ein Beitrag zur Dolichopidenmetamorphose. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 26: 319-332.
National Centers for Environmental Information (NCEI), National Oceanic and Atmospheric Administration (NOAA). 2024. Geographic reference maps. Retrieved August 23, 2024 from https://www.ncei.noaa.gov/access/monitoring/reference-maps/us-climate-regions.
Negrobov, O.P. and A.A. Stackelberg. 1971. Dolichopodidae. In Lindner, E. (ed.). Die Fliegen der Palaearktischen Region 284: 225-256 + pl. 13-28.*
Newton, N.H. 1984. Stem-boring insect larvae of Spartina alterniflora Loisel. (Poaceae): distribution, biology and influence on seed production. PhD thesis, North Carolina State University at Raleigh, 1984.
Palmer, M.W. 2016. Fly emerging from Tridens flavus stems. Contributor post at BugGuide.net. Retrieved August 6, 2024 from https://bugguide.net/node/view/1208426/.
PESI. 2024. Pan-European Species directories Infrastructure. Retrieved August 7, 2024 from https://www.eu-nomen.eu/portal/taxon.php?GUID=urn:lsid:faunaeur.org:taxname:136557.
Pitkin, B., Ellis, W., Plant, C., and R. Edmunds. 2024a. Thrypticus bellus Loew, 1869. In The leaf and stem mines of British flies and other insects. Retrieved August 7, 2024 from http://www.ukflymines.co.uk/Flies/Thrypticus_bellus.php.
Pitkin, B., Ellis, W., Plant, C., and R. Edmunds. 2024b. Thrypticus smaragdinus Gerstaecker, 1864. In The leaf and stem mines of British flies and other insects. Retrieved August 7, 2024 from http://www.ukflymines.co.uk/Flies/Thrypticus_smaragdinus.php.
Soreng, R.J., Peterson, P.M., Romaschenko, K., Davidse, G., Teisher, J.K., Clark, L.G., Barberá, P., Gillespie, L.J. and F.O. Zuloaga. 2017. A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications. Journal of Sytematics Evolution 55: 259-290. https://doi.org/10.1111/jse.12262
Strong, D.R., Lawton, J.H., and Sir Richard Southwood. 1984. Insects on Plants: Community Patterns and Mechanisms. Harvard University Press: Cambridge, MA.
U.S. Army Corps of Engineers. 2023. 2022 National Wetland Plant List, version 3.6. U.S. Army Engineer Research and Development Center, Vicksburg, MS. http://wetland-plants.usace.army.mil/
Waitzbauer, W., Pruscha, H., and O. Picher. 1973. Faunistisch-ökologische Untersuchungen an schilfbewohnenden Dipteren im Schilfgürtel des Neusiedler Sees. Sitzungsberichte der Akademie der Wissenschaften mathematisch-naturwissenschaftliche Klasse 181: 111-136.
Youtie, B.A., Stafford, M., and J.B. Johnson. 1987. Herbivorous and parasitic insect guilds associated with Great Basin wildrye (Elymus cinereus) in southern Idaho. Great Basin Naturalist 47(4): 644-651 (article 25).